

Flávia Regina Ferreira1; Bruna Ferrari2; Livia Mendes Sabia Acedo3; Juliana Emi Dias Ujihara4; Marcia Lanzoni de Alvarenga Lira5; Samuel Henrique Mandelbaum6
Keywords: MELANOMA; DIAGNOSIS; HISTOLOGY; IMMUNOHISTOCHEMISTRY
Cutaneous melanoma is a malignant tumor located in the dermal-epidermal junction of the skin, arising from the atypical transformation of melanocytes. It expresses a variety of phenotypes with different clinical and cytological variants, such as the extensive superficial, the nodular, the acral lentiginous and the lentigo maligna - which are the most classic - and the desmoplastic, the spitzoid and the amelanotic - which are the most unusual. 1-3
The desmoplastic melanoma (DM) is a rare variant characterized by invasive proliferation of spindle cells in the dermis and variable degrees of stromal deposition of collagen (desmoplasia). 1-3 It is more frequent in males of advanced age (mean age = 66 years) and with a history of chronic exposure to the sun, which may explain its prevalence in sun-exposed areas, especially the head and neck (53.2%). 2-4
The extremely variable and nonspecific clinical appearance means its diagnosis is challenging.The authors report an exuberant case of desmoplastic melanoma, atypical in appearance and at an unusual location, with an initial diagnosis of dermatofibrosarcoma.
An 83-year-old Caucasian male patient, a farmer by occupation, sought medical attention complaining of a "wound" located on the dorsum for eight months (Figures 1 and 2). The dermatological exam showed an erythematous tumoration in the lower left back, with firm consistency, and a bright and lobular surface, measuring about 15 cm at its longest axis. He described the onset as a small crusted papule that grew rapidly over the course of two weeks. There was an absence of local symptoms and palpable lymph nodes.
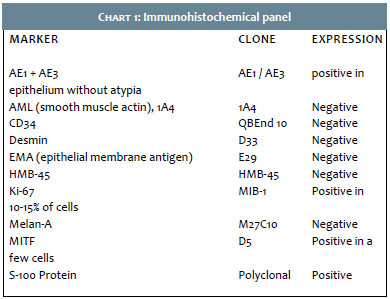
Once it was diagnosed as a dermatofibrosarcoma protuberans, the patient underwent an incisional fusiform biopsy. The pathology (Figures 3 and 4) suggested a spindle cell neoplasm at least 4.7 mm thick, involving the dermis and hypodermis. Based on this report, immunohistochemistry was carried out (Figure 5) evidencing negative HMB-45 and Melan-A, positive S-100, and a positive proliferative index (Ki67) in 10-15% of cells. The findings were compatible with the diagnosis of desmoplastic melanoma.
The patient was referred to the surgical oncology sector, undergoing a complete exeresis of the lesion and a new pathology, which confirmed the diagnosis of desmoplastic melanoma with a 14 mm Breslow, Clark V, one mitosis/mm mitotic index, absence of vascular and perineural invasion, and surgical margins free of neoplastic involvement. At the time this article was accepted for publication, the patient was under dermatologic and clinical oncologic follow up care.
First described in 1971 by Conley et al., the DM is a distinct and uncommon variant, representing less than 4% of cases of primary cutaneous melanoma, affecting two individuals per one million inhabitants. 1-4 It is characterized by a fibrous tumor constituted by spindle-shaped cells isolated in a dense fibrous matrix, producing or liberating collagen. The tumor often presents neurotropism, a growth pattern similar to that of the neuroma and neural differentiation - also described as neural transformation phenomenon.4 Unlike the non-desmoplastic melanomas, it presents a greater tendency for local growth and a lower tendency for lymph node metastasis. 4,5
Due to its extremely variable and nonspecific clinical appearance, the DM comprises a true diagnostic challenge. It commonly appears as a hypomelanotic or amelanotic nodule, papule or plaque, with firm consistency, affecting the dermis or even the subcutaneous, similarly to other fibrous lesions, which leads to misdiagnosis. The highest incidence of DM misdiagnosis occurs among the malignancies, with carcinomas, fibrosarcomas and amelanotic melanomas. Among the benign lesions, are the fibromatosis, the dermatofibroma, the melanocytic nevi and the scars.3,4,6,7 The association with intraepidermal atypical melanocytic proliferation - such as lentigo maligna - covering the lesion or present in the resection margins, has been reported, thus facilitating its recognition and diagnosis. 2,3,6,7 In the present description, the uncommon clinical presentation (lobular tumoration) and topography (lumbar region), the large proportions of the lesion (about 15 cm in its longest axis) and the initial suspicion of dermatofibrosarcoma protuberans rather than DM, reinforce the great variability and clinical non-specificity of this melanoma variant, justifying the possible diagnostic errors.
Dermoscopy has limited use in DM due to its variable clinical presentation, usual amelanotic appearance and the scarcity of information about its dermoscopic features.1,4 In 2008, Debarbieux et al. evaluated six patients with DM and only half of them showed positive dermoscopic criteria for melanocytic lesions. 8 Some of the predictive characteristics of DM in cases of amelanotic lesions are: the presence of signs of regression - such as scarring areas and "salt and pepper" appearance - as well as abnormal vascular patterns (serpentine, dotted and/or speckled), and pink/milky-red areas. 1,4,8
Histologically, it presents a spindle cell infiltrate with mild to marked nuclear atypia, invading the dermis and the subcutaneous tissue. They are arranged in variable patterns of desmoplasia, neurotropism and neural differentiation.3,4 Staining with hematoxylin and eosin may be insufficient for diagnosis, since the tumor cells are almost always depigmented, which calls for an immunohistochemical study. The S-100, neuron specific enolase and vimentin markers are positive in 95% of cases, with the vast majority of DMs being negative for HMB-45 and Melan A.3 In a relatively recent study of a series of 11 DM patients, the presence of diffusely positive marking was demonstrated in 100% of the cases with the use of WT1, a potential marker for this clinical type.9 Dense intratumoral lymphocyte aggregates can also be very commonly present, in addition to in situ melanoma.4
At diagnosis, it has an average thickness of 2.5 mm to 6.5 mm, reaches the reticular dermis, and is most often classified as Clark IV-V,6 aligned with the findings of the clinical case in question - except for the latter, which had a surprising thickness of 14 mm.
Based on the degree of desmoplasia, the DM was classified into two histological subtypes: pure or combined. The pure subtype relates to minor lymph node involvement, less aggressive clinical course and more than 90% of desmoplastic compromise, while the combined subtype has less than 90% desmoplastic involvement, thick cell tumor focus without fibrosis, with irregular nuclei and higher rate of mitosis. 1,4
The role of the sentinel node in DM is not clear, and its routine use is not recommended. Some authors advocate the importance of staging cases with 1 mm or more, while others avoid this practice due to the lower tendency for lymph node metastasis. Other indications for sentinel lymph node biopsy are: the mixed subtype, the presence of neurotropism, ulceration, and a high mitotic rate. 2,10
Wide and early surgical resection is the treatment of choice. In lesions of 1- 2 mm thick, a 2 cm margin is recommended, while in lesions of over 2 mm, a 2 cm margin is mandatory. Adjuvant radiation therapy has shown beneficial effects in cases of local recurrence, excision with narrow margins, residual tumors and neural involvement. Systemic metastases were observed in 7-44% of DM cases with the lung, liver, and bones being the organs most commonly affected. In such cases, Ipilimumab and Vemurafenib are therapeutic options, however their effectiveness has not been fully proven. 1,4,6
In conclusion, the unusual clinic-pathological presentation and controversies in the diagnosis, staging and treatment of the DM make it a challenging diagnosis for both dermatologists and dermatopathologists. Additional studies are necessary for a better understanding and management of this variant.
1. Chen LL, Jaimes N, Barker CA, Busam KJ, Marghoob AA. Desmoplastic melanoma: A review. J Am Acad Dermatol. 2013;68(5):825-33.
2. Ashwin Alva K, Rajeshwara KV, Udaykumar. Desmoplastic Melanoma: A Diagnostic Dilemma. J Clin Diagn Res. 2013;7(6): 1172-3.
3. Bastos-Junior CS, Piñeiro-Maceira JM, Moraes FMB. Melanoma desmoplásico associado a lesão lentiginosa intraepidérmica, com evolução de 10 anos: relato de caso e revisão bibliográfica. An Bras Dermatol. 2013;88(3):413-8.
4. Paschoal FM, Yamada VL, Enokihara MMSS, Filho CDSM. Melanoma Desmoplásico. Surg Cosmet Dermatol. 2012;4(1):1-7.
5. Conley J, Latterly R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer. 1971; 28(4): 914-36.
6. Carlson JA, Dickersin GR, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma: a clinicopathologic analysis of 28 cases. Cancer. 1995;75(2):478-94.
7. Egbert B, Kempson R, Sagebiel R. Desmoplastic malignant melanoma: a clinicohistopathologic study of 25 cases. Cancer. 1988;62(9):2033-41.
8. Debarbieux S, Ronger-Salve S, Dalle S, Balme B, Thomas L. Dermoscopy of desmoplastic melanoma: report of six cases. Br J Dermatol. 2008;159(2):360-3.
9. Wilsher M, Cheerala B. WT1 as a complementary marker of malignant melanoma: an immunohistochemical study of whole sections. Histopathology. 2007;51(5):605-10.
10. Pawlik TM, Ross MI, Prieto VG, Ballo MT, Johnson MM, Mansfield PF, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106(4):900-6.
This study was performed at the Hospital Universitário de Taubaté (HUT) - Taubaté (SP), Brazil.